406
Views & Citations10
Likes & Shares
Choriocarcinoma can be classified into two distinct types: gestational and non-gestational choriocarcinoma and is characterized by elevated levels of beta-human chorionic gonadotropin in the blood produced by the malignant trophoblastic cells. Around 25% of gestational choriocarcinoma cases arise from the transformation of a molar pregnancy, while 50% can occur after a full-term pregnancy. Choriocarcinoma can manifest months or even several years after a prior pregnancy, often affecting individuals of their childbearing age [2]. Choriocarcinoma presents diverse clinical manifestations, including abnormal vaginal bleeding and metastatic symptoms [3].
Metastasis of choriocarcinoma is commonly observed in the lungs and occasionally in the brain. The success of chemotherapy varies depending on the stage of choriocarcinoma, with higher-stage cases necessitating a combination of surgery and additional chemotherapy [4]. The prognosis of gestational choriocarcinoma is generally more favorable when compared to the non-gestational form, primarily due to the positive outcomes achieved through multiple-agent chemotherapy regimens [3].
CASE REPORT
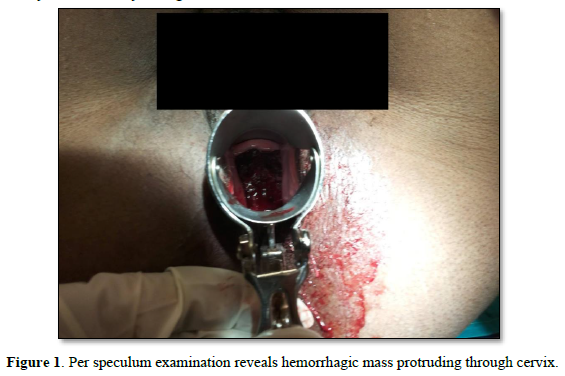
A 43-year-old P2L2A1, presented to the gynecology office with minimal continuous bleeding for two months. She had a spontaneous 8-week abortion one year ago. Her ongoing bleeding was painless, with minimal flow preceded by regular menstrual cycles. There was no history of molar pregnancy, recurrent abortions, or hormonal medication intake in the past. She never used contraception in her lifetime. Her family had no history of inherited cancers or genetic disorders. She attained menarche at age 12. Her past menstrual cycles were regular on 3/28, painful, with moderate flow. Both deliveries were full-term spontaneous vaginal deliveries ten years and five years ago. She had no previous medical or surgical history. In the past, there was no history of food or drug allergies or substance abuse.Her vitals were normal. Chest auscultation revealed normal vesicular breath sounds and S1 and S2. Physical examination revealed that the rest of the organ systems, such as respiratory, neurological, cardiovascular, gastrointestinal, urinary, and bilateral extremities, were normal. Her abdomen was soft with no organomegaly or free fluid. A speculum examination revealed a flat, irregular fragile mass measuring >4cm along the cervical canal (Figure 1).
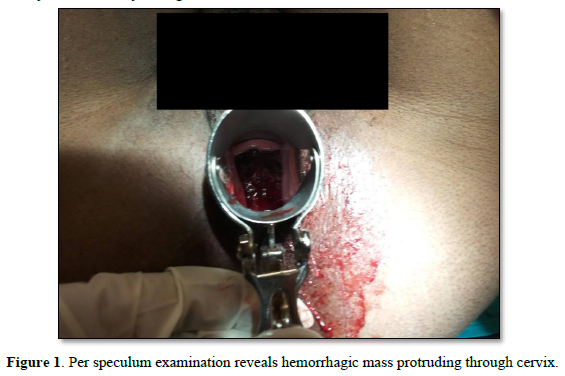
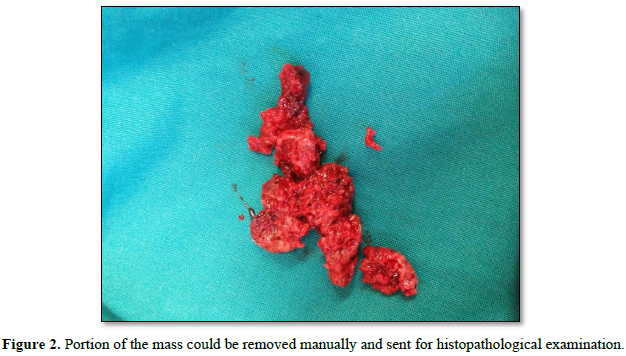
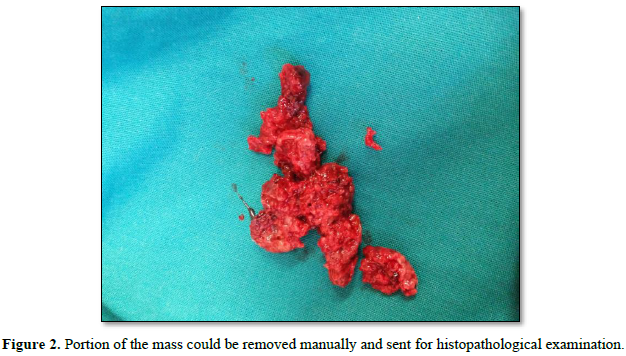
The external cervical rim was intact, in addition to bleeding on touch. Bimanual examination revealed an anteverted, bulky, and freely mobile uterus and fornices free. The mass was fragile, soft in consistency, hemorrhagic, with a broad base pedicle, and could be removed digitally (Figure 2).
Per rectal examination revealed a mobile bulky cervix and uterus and no uterosacral nodularity.
The urine pregnancy test was positive several times during the evaluation. Ultrasonography pelvis report suggested uterine fibroid with no evidence of adnexal pathology or free fluid. Hematological investigations were normal limits. Human chorionic gonadotropin titers increased weekly with a baseline value of 1,57,500 mIU/ml. No abnormalities were detected on the chest x-ray. While the patient was waiting for her computed tomography appointment for metastasis evaluation, her histopathological results revealed the presence of areas of abnormal intermediate trophoblast and cytotrophoblast, surrounded by syncytiotrophoblasts and absent chorionic villi confirming choriocarcinoma. She was referred to an oncology tertiary care center for further management.
DISCUSSION
A gestational trophoblastic disease is a diverse group of pregnancy-related disorders distinguished by abnormal growth of trophoblastic tissue. With the advent of first-trimester ultrasounds, the clinical presentation has shifted towards earlier detection. While many patients remain asymptomatic, vaginal bleeding is the predominant initial symptom in most cases [5]. In addition to postpartum abnormal vaginal bleeding, choriocarcinoma can manifest with various clinical presentations. These may include bleeding from metastatic sites such as the liver, spleen, intestines, lung, or brain, as well as pulmonary symptoms and neurological signs resulting from metastasis to the spine or brain [3].
Approximately 50% of choriocarcinoma cases stem from preexisting complete hydatidiform moles. Spontaneous abortions contribute to 20% of the remaining cases, while ectopic pregnancies represent approximately 2.5%-3%. Normal pregnancies account for 25%-30% of choriocarcinoma occurrences [6]. In North America and Europe, the incidence of choriocarcinoma is reported to be approximately 1 in 40,000 pregnancies. On the other hand, in Southeast Asia and Japan, the incidence rates are notably higher, with figures of 9.2 and 3.3 per 40,000 pregnancies, respectively [3].
Only a few studies are reported in the literature on cases of choriocarcinoma following a nonmolar spontaneous abortion. In a study of data on 100 women with choriocarcinoma between 1985 and 1994, 32 were preceded by a nonmolar abortion. Of these cases mortality rate was 21% after a live birth versus 6% after a nonmolar abortion [7].
In cases where choriocarcinoma tissue was analyzed using short tandem repeat genetic test, a study revealed that nonmolar pregnancy, without any prior history of molar pregnancies, was identified as the underlying cause of high-risk gestational trophoblastic neoplasia [8]. Occasionally, gestational trophoblastic neoplasia may occur after an ectopic molar pregnancy [9]. A pure non-gestational ovarian choriocarcinoma has been documented [10]. A study described choriocarcinoma syndrome in a patient who died of hemorrhagic hepatic metastatic lesions. The histopathology of the abdominopelvic mass revealed choriocarcinoma from an undescended testis [11].
Performing routine microscopic examinations on all placentas and products of conception can enhance the accurate detection of intraplacental choriocarcinoma and improves management strategies as described in a study [12]. Our patient with a positive pregnancy test reported continuous bleeding for two months, where our provisional diagnosis was incomplete abortion before the clinical examination. Similar to our case presentation, a study describes a postpartum woman with continuous bleeding for five months with choriocarcinoma treated with multi-agent chemotherapy [13].
Limitations of our report include an inadequate evaluation and follow-up of the patient due to the heavy patient turnover at the district hospital. Histopathological confirmation remains the gold standard investigation for diagnosing choriocarcinoma [14].
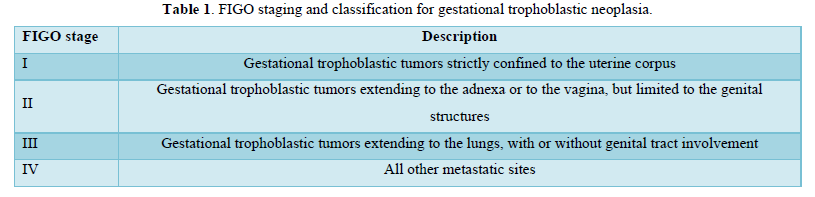
Table 1 describes the FIGO staging and classification for gestational trophoblastic neoplasia.
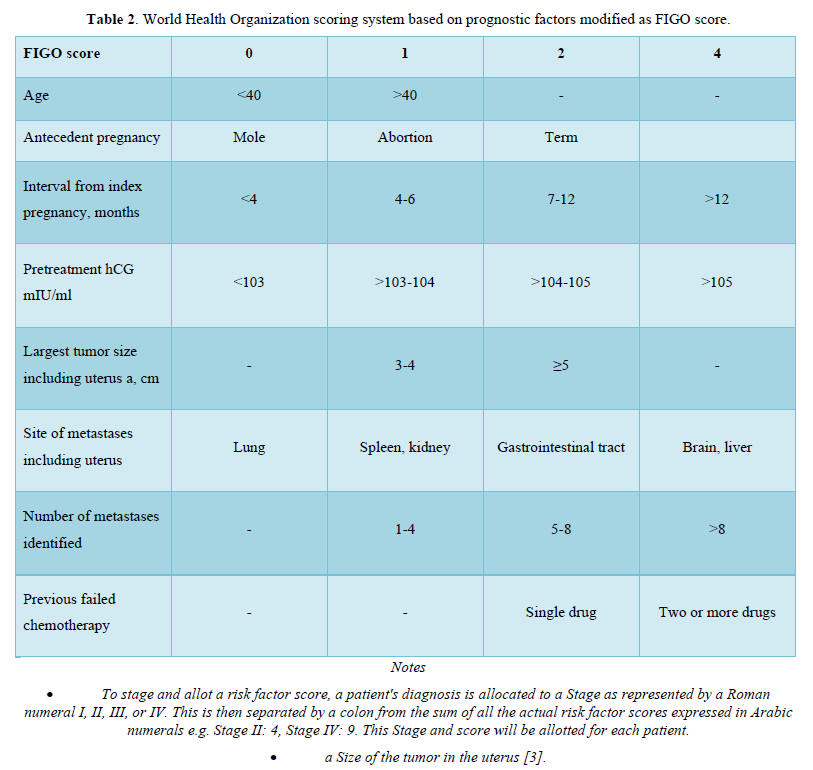
Table 2 describes World Health Organization scoring system based on prognostic factors modified as FIGO score.
Table 3 details the Protocol for Treatment of GTN.
Consideration to preserve fertility comes foremost in-patient management with low-risk gestational trophoblastic neoplasia. A study revealed that fertility-preserving surgeries performed for placental site trophoblastic tumor, hysteroscopic resection, laparoscopic resection, and uterine evacuation were considered a safe therapeutic choice in women of the childbearing age group [16].
For low-risk cases of choriocarcinoma, fertility-preserving procedures, chemotherapy, and hysterectomy are employed for selected patients. Choriocarcinoma metastasizes primarily to the lungs, with a lesser propensity for spreading to the brain. In an analysis spanning from 1985 to 2013, it was observed that for Stage I choriocarcinoma, 83% of patients were successfully cured through the use of single-agent chemotherapy. The remaining 17% achieved complete remission with the help of additional chemotherapy or hysterectomy. In the case of Stage II through IV choriocarcinoma, all patients were cured; however, it necessitated the combination of surgery and additional chemotherapy [4].
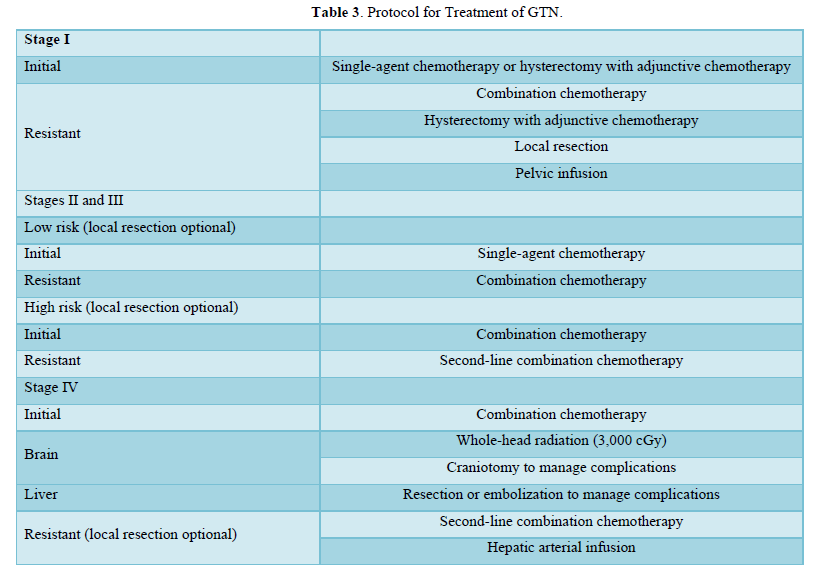
A higher risk score of 5-6 and a clinicopathologic diagnosis of choriocarcinoma are correlated with an elevated risk of developing resistance to single-agent chemotherapy. High-risk gestational trophoblastic neoplasia is effectively treated using multiple-agent chemotherapy regimens. The widely utilized regimen is EMA-CO, which combines etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine. Notably, the overall survival rates for patients with high-risk gestational trophoblastic neoplasia have reached impressive levels, with rates as high as 95% [3]. Few studies revealed successful pregnancy outcomes in stage III gestational trophoblastic neoplasia cases and metastatic choriocarcinoma after multi-agent chemotherapy [17,18].
CONCLUSION
Gestational trophoblastic neoplasia, particularly choriocarcinoma, presents with varied clinical manifestations, including abnormal vaginal bleeding and metastatic symptoms. To provide optimal care to patients with gestational trophoblastic disease, healthcare professionals must stay up-to-date with the latest diagnostic and management guidelines.
The success of chemotherapy varies depending on the stage of choriocarcinoma, with higher-stage cases requiring a combination of surgery and additional chemotherapy. Understanding the various factors and presentations associated with choriocarcinoma is crucial for accurate diagnosis, effective treatment, and improved patient outcomes. Optimally, the active management of gestational trophoblastic disease (GTD) calls for a multidisciplinary team approach comprising specialists in gynecology, gynecological oncology, medical oncology, pathology, as well as midwives, nurses, and experts from the hCG laboratory. This collaborative approach ensures comprehensive and well-coordinated care for GTD patients.
- Stabile G, Scalia MS, Stampalija T, Bruno M, Laganà AS, et al. (2023) Placental Cholangiocarcinoma a Specific Histological Pattern of Uncertain Incidence and Clinical Impact: Systematic Review of the Literature. J Clin Med 12(9): 3065.
- De Lucia DR, Castaldo A, D'Agostino V, Ascione R, Pesce I, et al. (2021) Metastatic choriocarcinoma with hemorrhagic complications and spontaneous ovarian hyperstimulation syndrome: A case report. Radiol Case Rep 16(12): 3868-3874.
- Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, et al. (2021) Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet 155(1): 86-93.
- Prabhu IK, Rosenbaum A (2021) Hydatidiform Mole in a Patient with a Concern for Neoplasia: A Case Report. Cureus 12(9): e10319.
- Lok C, Frijstein M, van Trommel N (2021) Clinical presentation and diagnosis of Gestational Trophoblastic Disease. Best Pract Res Clin Obstet Gynaecol 74: 42-52.
- Soper JT, Mutch DG, Schink JC (2004) American College of Obstetricians and Gynecologists. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol Oncol 93(3): 575-585.
- Tidy JA, Rustin GJ, Newlands ES, Foskett M, Fuller S, et al. (1995) Presentation and management of choriocarcinoma after nonmolar pregnancy. Br J Obstet Gynaecol 102(9): 715-719.
- Nishino K, Yamamoto E, Oda Y, Watanabe E, Niimi K, et al. (2021) Short tandem repeat analysis to identify the causative pregnancy of high-risk gestational trophoblastic neoplasia: Molar versus nonmolar pregnancy and its relation to the outcome. Placenta 112: 28-35.
- Dollinger S, Yeoshoua E, Eitan R (2021) A rare case of gestational trophoblastic neoplasia following an ectopic molar pregnancy. Gynecol Oncol Rep 37: 100798.
- Rao KV, Konar S, Gangadharan J, Vikas V, Sampath S (2015) A pure non-gestational ovarian choriocarcinoma with delayed solitary brain metastases: Case report and review of the literature. J Neurosci Rural Pract 6(4): 578-581.
- Baagar K, Khan FY, Alkuwari E (2013) Choriocarcinoma syndrome: A case report and a literature review. Case Rep Oncol Med 2013: 697251.
- Caldas RF, Oliveira P, Rodrigues C, Reis I, Scigliano H, et al. (2017) Intraplacental Choriocarcinoma: Rare or Underdiagnosed? Report of 2 Cases Diagnosed after an Incomplete Miscarriage and a Preterm Spontaneous Vaginal Delivery. Case Rep Med 2017: 7892980.
- Katsanevakis E, Oatham A, Mathew D (2022) Choriocarcinoma After Full-Term Pregnancy: A Case Report and Review of the Literature. Cureus 14(2): e22200.
- Neelam, Verma M, Agarwal M (2020) Primary uterine choriocarcinoma presented as suspected ectopic pregnancy: A case report. Int J Clin Obstet Gynaecol 4(2): 161-162.
- Berek JS, Berek DL (2020) Berek and Novak’s Gynecology. Wolter Kluwer, Philadelphia, PA.
- Chiofalo B, Palmara V, Laganà AS, Triolo O, Vitale SG, et al. (2017) Fertility Sparing Strategies in Patients Affected by Placental Site Trophoblastic Tumor. Curr Treat Options Oncol 18(10): 58.
- Bhonsale A, Fonseca M (2012) Successful pregnancy after chemotherapy for choriocarcinoma. J Obstet Gynaecol India 62(2): 197-198.
- Behtash N, Behnamfar F, Hamedi B, Ramezanzadeh F (2009) Term delivery following successful treatment of choriocarcinoma with brain metastases, a case report and review of literature. Arch Gynecol Obstet 279(4): 579-581.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)